Covid-19 Technical Bulletin

Contents
As one of the first commercial labs in the United States to test for COVID-19, Streamline Scientific utilizes PCR technology to deliver fast, accurate results to help inform and safeguard our communities. Streamline Scientific is operating around the clock to meet demand and produce quick results.
What is the Sensitivity & Specificity of your test?
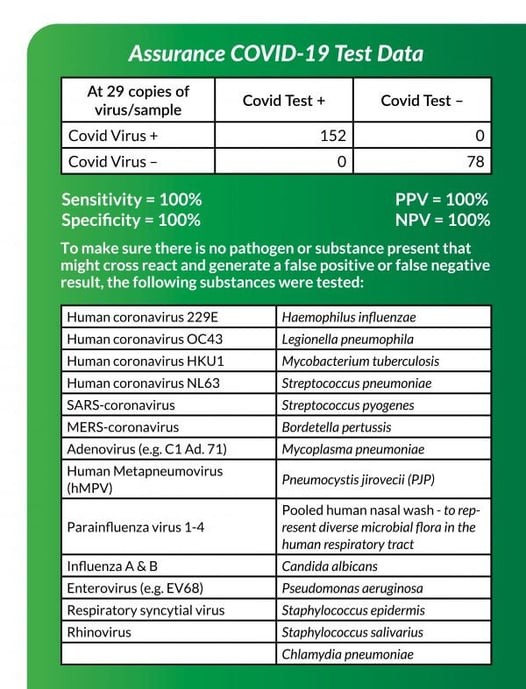
On the bottom right, is a 2×2 table showing the data we submitted to the FDA. Test validation is performed under ideal conditions contrived to replicate or represent actual sample processing. To accomplish this, we spiked real saliva and nasal mucosa samples with virus provided to us from the CDC’s agent. These samples were processed as a real patient sample which serves as true positive samples. In addition, many other bacteria and viruses common in the respiratory tract were tested to make sure there was no cross reactivity. These serve as true negative samples.
Our data does not show any false positive or false negative results. Again, these were performed under ideal conditions. This does not mean false positives and negatives will not happen. False negative results are more likely than false positives. False positive results can really only result from contamination or degradation of the assay. Each sample is run against positive and negative controls to detect these making this very unlikely. We regularly check for contamination in the lab and decontaminate after each sample to prevent any carryover from previous samples. The assay can degrade resulting in a false signal which has the potential for the machine to interpret as a positive result. However, these are easily recognized upon re-inspection. Our test results are interpreted by 2 sets of human eyes before approving. In addition, our assays are prepared fresh weekly and not stored.
False negative results are more likely and almost exclusively result from specimen collection. Our test can detect as few as 1-5 copies of viral RNA most of the time. It will detect 30 copies 100% of the time. As with any infection, the pathogen is usually most concentrated at the point of infection or inflammation. The goal is to swab the area of infection or inflammation to get the best sample. When there is no obvious inflammation or the patient has no symptoms, the default is usually an NP swab because the virus is usually more concentrated there.
If the sample is near the limit of detection (a few copies), then it could easily be positive on our test and negative on another test. The test can be negative the next day upon re-sampling.
When we knew PPE and swabs were in short supply, we determined that mid-turbinate and anterior nares were as good as an NP swab. United Healthcare performed a study documenting this. As a result, this allowed for self-collection. This does not mean that NP, mid turbinate, and anterior nares swabs are 100%. It all depends on where the infection is located and did the swab get it. A more sensitive PCR test has a better chance of detecting it.
We have seen this virus remain detectable for 50+ days with the typical range around 3 weeks; however, in some samples, it was gone the next day. We have also seen this virus become detectable weeks later after being negative.
What “rapid test” should I order?
There is confusion using the term “rapid test.” The proper distinction to make is between a point-of-care versus an in-lab test. Current testing methods to detect COVID-19 include culturing, PCR, and antibody/antigen testing.
Culturing is not an option for this virus for many reasons and has been prohibited for COVID-19 use.
Antibody and antigen testing are generally rapid and available as a CLIA waived point of care test. Antibody and antigen testing can be put together via lateral flow paper chromatography cassettes which can be used as a point-of-care test with results in 10-20 minutes. Many people consider this as the “rapid test”. These tests tend to have poor sensitivity—typically 60-70%. These tests require an antibody response which takes days and makes this test not useful for detecting an infection.
PCR is the gold standard and is generally considered a highly complex test performed in a lab. PCR identifies the pathogen genetically and is very sensitive and specific. This is the only test that can accurately and timely identify the pathogen. Our PCR lab can test over 400 samples per hour and detect as few as 1-5 copies of RNA per sample.
There are a few point-of-care tests that utilize PCR technology. These point-of-care PCR tests can run 1 test at a time to detect a single pathogen, and it takes 15 to 90 minutes to run depending on the machine. These tests require the purchase of a machine and proprietary consumables to operate which make it somewhat expensive. These devices tend to have a higher level of detection, usually 100-200 copies of RNA per sample. While these are convenient and quite good, if the sample contains a lower amount of pathogen, there is a greater chance it will get missed. We use commercial machines from very well established companies, Bio-Rad and Thermofisher. In addition, we employ automation in order to limit human error which can be significant when handling very low volumes and when introducing contamination.
The Streamline Scientific Process
- Easy Ordering – Choose from any of our panels or select individual pathogens.
- Sample Collection – Our clients will be stocked with lab supplies, requisition forms, and specimen bags with customer service information and instructions on sample collection/shipping.
- Fast, Accurate Testing – We use PCR testing to accurately identify the patient’s specific infection with results back quickly.
- Easy to Understand Lab Reports – These reports show infections and guide clinicians to the appropriate treatment.
- Insurance/Billing – We will bill the patient’s insurance. Patients can contact us directly with any billing questions.
This test detects the presence of pathogen only and does not detect disease. Clinically correlate results for significance.
Ready to take charge of your lab
services?
START YOUR LAB
USE OUR LAB
© Copyright 2025 | All Rights Reserved | Privacy Policy | Terms of Use | Do not sell or share my personal information