Vaginal Infections and Maternal-Child Health
Contents
Relationship Between Vaginal Microbiota and Infections
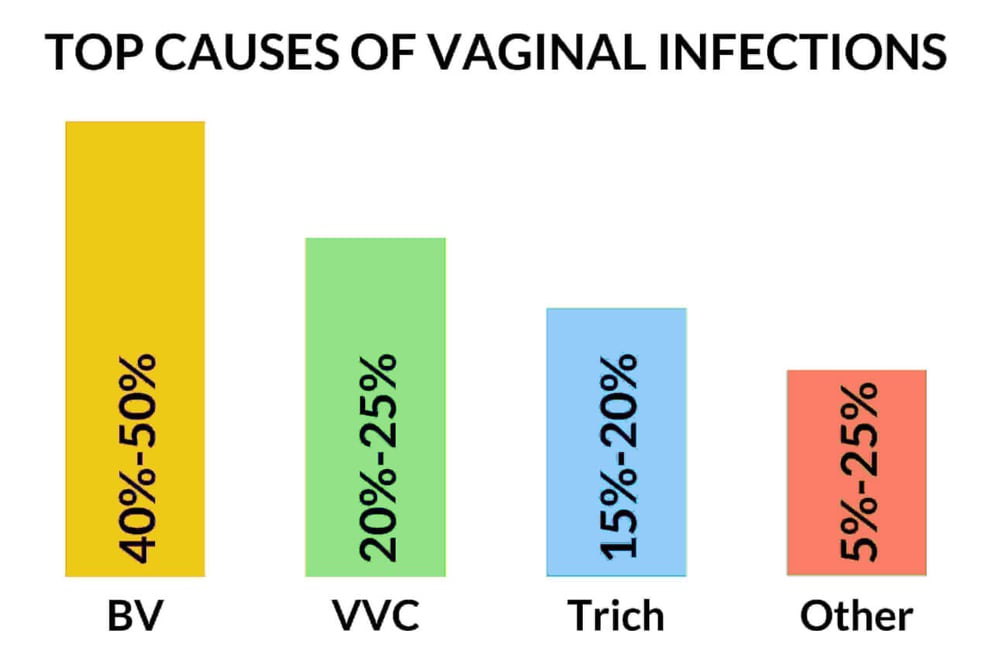
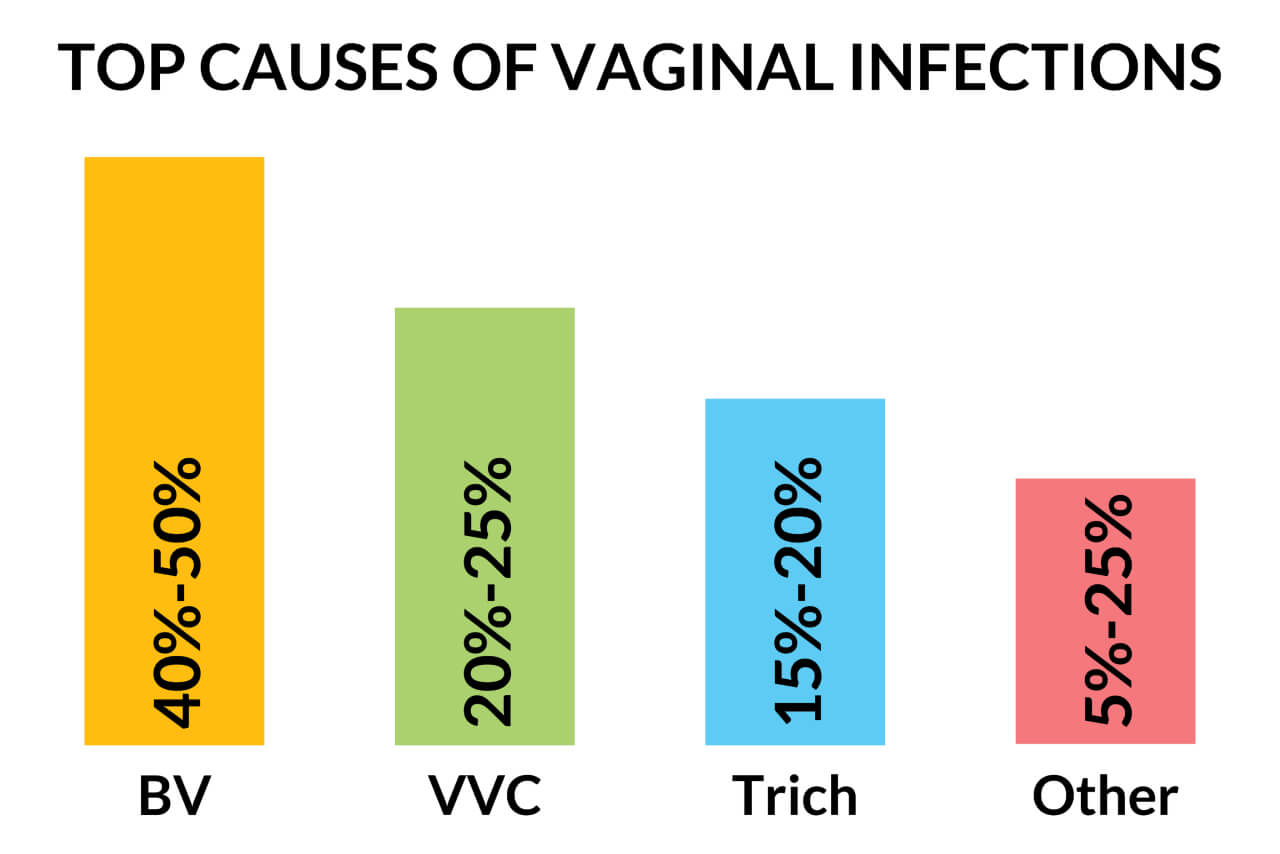
The vaginal microbiota plays a central role in the health and disease of the female genital tract.28 Vaginal infections result from an imbalance in the normal vaginal microbiota and often lead to symptoms such as itching, burning, increased vaginal discharge, foul or fishy odor, and dysuria. Vaginal discharge is most commonly due to bacterial vaginosis (BV) and infections with Trichomonas vaginalis (T. vaginalis) and Candida species. BV accounts for 40% to 50% of cases of vaginal infections in which a cause is identified, vulvovaginal candidiasis (VVC) accounts for 20% to 25%, and trichomoniasis for 15% to 20% of cases.33
Alterations in the normal vaginal microflora have also been associated with an increased susceptibility to sexually transmitted and other pathogenic organisms that lead to adverse reproductive outcomes ranging from infertility and chronic pelvic pain to significant maternal and neonatal morbidity and mortality.24 Therefore, accurate diagnosis and targeted treatment of vaginal infections are paramount for preventing a wide range of poor health outcomes for women.
Composition of a Normal Healthy Vaginal Microbiota
Over the past several years, nucleic acid-based testing has shed considerable light on the composition of normal vaginal microbiota and its role in preventing infections. A normal, healthy vaginal microbiome consists of numerous microbes that work synergistically to inhibit the growth of potentially pathogenic organisms.
An abundance of several species of Lactobacillus plays a central role in maintaining a healthy microbiome. The production of lactic acid and hydrogen peroxide by a number of Lactobacilli helps to maintain a low vaginal pH30, and hydrogen peroxide is considered essential for inhibiting the overgrowth of normal facultative anaerobes.11 Lactobacillus species also produce bacteriocins, biosurfactants, and co-aggregating molecules that inhibit the adhesion of pathogens.36 However, not all Lactobacilli are created equal. The anti-inflammatory immune profile linked to the presence of Lactobacillus crispatus seems to protect women from disruptions of the normal vaginal microbiome.13, 42 For instance, women with Lactobacillus crispatus-dominated vaginal microbiome were found to have no bacterial sexually transmitted infections as well as the lowest prevalence of human immunodeficiency virus (HIV), human papillomavirus (HPV), and herpes simplex virus type 2 (HSV- 2).6 In vitro studies have also demonstrated an inhibitory effect of vaginal Lactobacilli on the growth of Neisseria gonorrhoeae (N. gonorrhoeae).41
The normal vaginal microbiota also includes numerous commensal microorganisms such as Candida albicans (C. albicans), Staphylococcus aureus (S. aureus), and Streptococcus agalactiae (S. agalactiae or group B Streptococcus (GBS)) that under normal conditions exist symbiotically with other organisms within a healthy microbiome.19
The composition of normal vaginal microbiota is heavily influenced by numerous factors including age, ethnicity, changes in hormonal levels, environmental and behavioral factors, which must all be taken into consideration when evaluating test results.
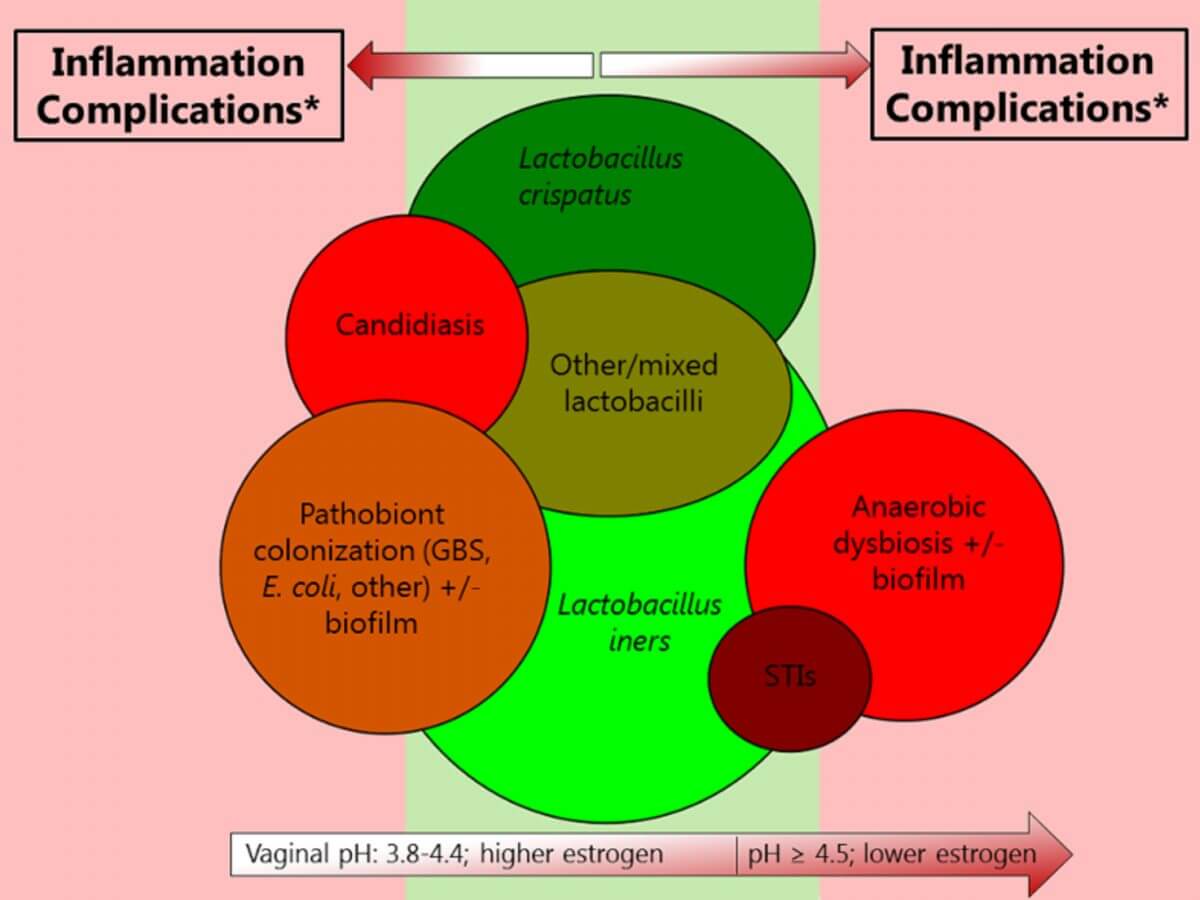
Figure 1. Visualization of interrelationships among various urogenital conditions involving micro-organisms.42
Green colors indicate desirable conditions, and red colors indicate undesirable conditions. In both cases, the darker the color, the more desirable or undesirable the condition, respectively. The size of the circles is relative to the size of the respective epidemics, but only very roughly. The STI circle does not include viral STIs. The circles on the far left and far right appear as if they do not overlap because the image is two-dimensional, but they do overlap somewhat. It is important to note that few studies on the associations between urogenital conditions and host responses or adverse outcomes (which determine whether a condition is desirable or undesirable) have been holistic. For example, many studies only employ 16S ribosomal RNA sequencing of the vaginal microbiota, but this does not cover fungi, protozoa, and viruses and does not reliably identify Chlamydia trachomatis and Neisseria gonorrhoeae. Abbreviations: GBS, Group B streptococcus; STI, sexually transmitted infection. * Complications include HIV acquisition, pelvic inflammatory disease, adverse pregnancy outcomes, and maternal and neonatal infections.42
Vaginal Dysbiosis: Disruption of the Normal Microbiota Leads to Adverse Reproductive Health Outcomes
Vaginal dysbiosis is defined as a prolonged deviation from a low-diversity, Lactobacilli-dominated vaginal microbiome. Bacterial vaginosis (BV), aerobic vaginitis (AV), and infections with T. vaginalis are all associated with vaginal dysbiosis, while vaginal colonization with Candida species appears to be more common in women with Lactobacilli-dominated microbiome.4, 20, 45 Vaginal dysbiosis disrupts the cervico-mucosal barrier such that mucus and vaginal secretions are less efficient in their ability to trap or inactivate pathogens.6
The most common type of vaginal dysbiosis is high-diversity anaerobic dysbiosis as seen with bacterial vaginosis (BV) in which disruption in Lactobacilli dominance leads to an overgrowth of anaerobic bacteria including Gardnerella vaginalis (G. vaginalis), Atopobium vaginae (A. vaginae), Prevotella species, Mobiluncus species, Megasphaera species, Ureaplasma species, Mycoplasma species and numerous other fastidious anaerobes.9, 43
BV-associated anaerobic dysbiosis also increases the risk of infection with HSV-2, C. trachomatis, and N. gonorrhoeae2, 10 as well as HPV, HIV, and T. vaginalis infection.45 BV has not only linked the development of ascending pelvic inflammatory disease32, but BV-associated organisms have also been directly implicated in infections such as acute salpingitis and endometritis.16 Obstetrical complications such as preterm delivery and pre-labor rupture of membranes PROMs are more common in women with BV and are potentially mediated through the activation of inflammatory pathways involved in the initiation of labor.3, 19
Aerobic vaginitis (AV) is thought to result from an underlying inflammatory disorder with the secondary development of decreased Lactobacilli and overgrowth of aerobic bacteria such as GBS, Enterococcus faecalis (E. faecalis), Escherichia coli (E. coli), and S. aureus.25, 37 The incidence of AV is between 5% and 23% of reproductive-age women12 and 4-8% of pregnant women.35 In the absence of a proper diagnostic evaluation, AV may be misdiagnosed as BV. If undiagnosed, untreated or incorrectly treated, AV can lead to complications such as desquamative inflammatory vaginitis and has also been linked to preterm birth (PTB), PROM, chorioamnionitis, funisitis of the newborn, and neonatal ICU admissions.17, 22
Bacterial pathobionts comprise another type of vaginal dysbiosis.44 Pathobionts are defined as any potentially pathogenic organism that, under normal circumstances, causes no harm. In the vaginal microbiome, pathobionts include GBS, S. aureus, and species in the Enterobacteriaceae family among others.18, 44 These bacteria are often associated with the development of pelvic inflammatory disease as well as severe maternal and neonatal infections.5, 8 GBS and E. coli are associated with preterm birth, very-low-birth-weight delivery, and puerperal sepsis1, 23, and are the leading cause of early-neonatal sepsis worldwide.40
Nucleic Acid Testing for Vaginal Infections
History and physical examination cannot be relied upon to make a diagnosis of vaginal infections. Overall, clinical diagnosis of BV, trichomoniasis, and Candida vaginitis based on physical examination and microscopy has been shown to be 81- 85% sensitive and 70-99% specific.26 Therefore, accurate and comprehensive diagnostic methods are needed to direct treatment and reduce risks of recurrent or more severe vaginal infections and their broader sequelae. Nucleic-acid based tests have demonstrated greater accuracy than standard office-based tests such as microscopy that can be labor intensive and susceptible to subjective interpretation, and laboratory tests such as culture and Gram stain that can be time-consuming and delay results.
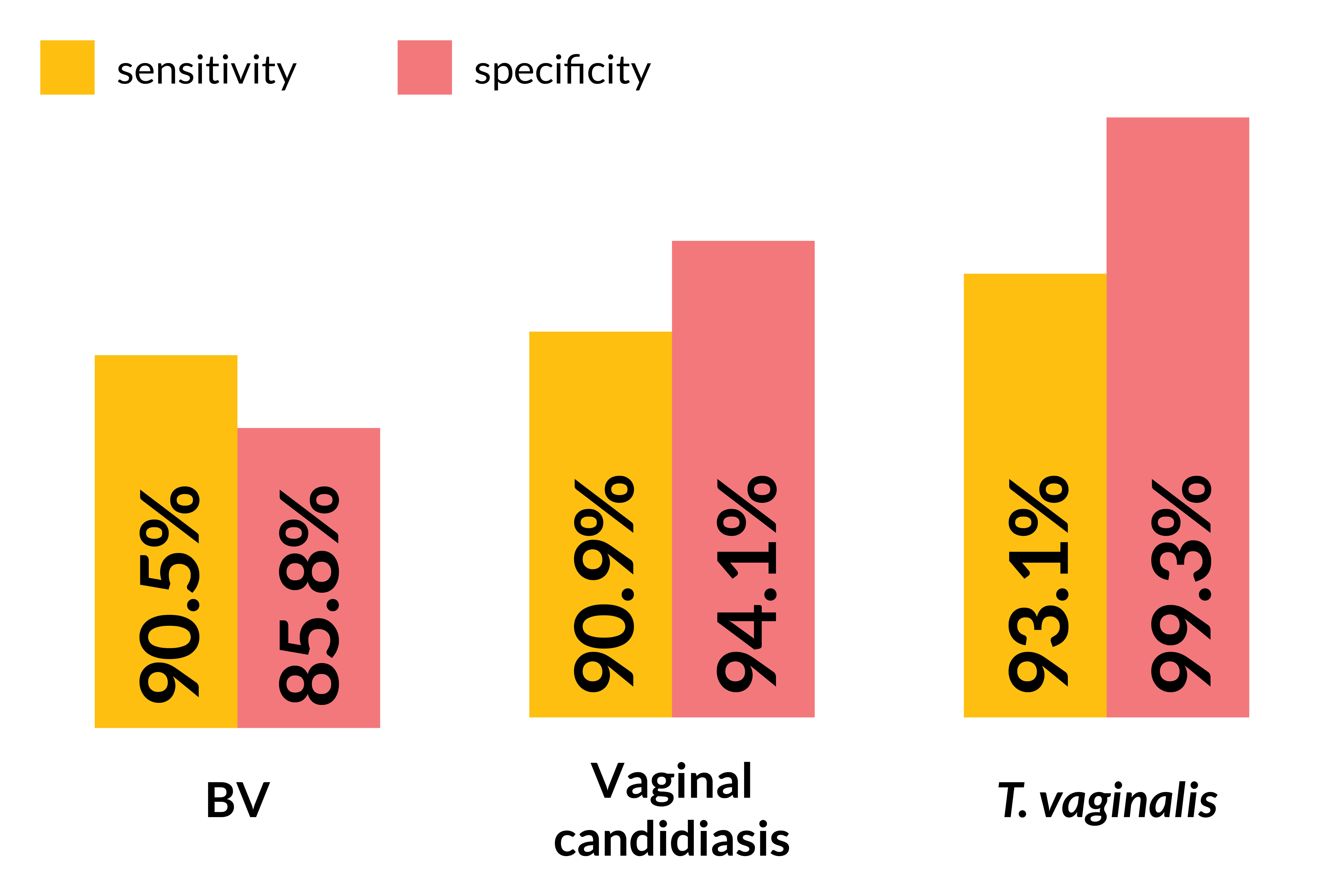
In a study evaluating 1,740 clinician- and self-collected samples from symptomatic patients, the sensitivity and specificity of nucleic acid amplification compared with the reference methods (including Gram stain, microscopy, Amsel criteria, and culture) was 90.5% and 85.8% for BV, 90.9% and 94.1% for vaginal candidiasis, and 93.1% and 99.3% for the detection of T. vaginalis, respectively.14
Studies have shown that molecular diagnostic methods are more accurate and objective than microscopy for diagnosing BV.27, 38 The Amsel criteria-based on the evaluation of thin, discharge, positive whiff test, clue cells on microscopy—is often used in office settings and has sensitivities ranging from 83 to 93% and specificities from 82 to 94%.39
Compared to Amsel criteria and the Nugent score, quantitative real-time PCR assay laboratory testing G. vaginalis has demonstrated a sensitivity and specificity of 100 and 93%, respectively.29 The use of a comprehensive PCR assay may also provide additional diagnostic benefits. For instance, in one study, detection of a high prevalence of AV organisms using a quantitative PCR assay suggested the possibility of alternative diagnoses to BV.27 Furthermore, the ability to measure the relative abundance of normal and BV microbiota versus determining an absolute count of BV-related microorganisms may provide a more accurate determination of BV status.27, 38
Currently, the Centers for Disease Control and Prevention (CDC) recommends nucleic acid amplification testing for the diagnosis of trichomoniasis in symptomatic or high-risk women because of the higher sensitivity than saline microscopy (95% to 100% vs. 51% to 65%). In addition, these tests can be performed on endocervical, vaginal, or urine specimens, or on liquid-based Pap test samples.33
Ninety-five million infections with C. trachomatis and N. gonorrhoeae are estimated to occur globally, and 15% of untreated infections progress to pelvic inflammatory disease.15, 34 Therefore, cervical or vaginal nucleic acid amplification tests for N. gonorrhoeae and C. trachomatis infection should be performed in all women with suspected pelvic inflammatory disease.8
Testing for Antibiotic Resistance
A growing number of opportunistic pathogens demonstrate antibiotic resistance. Treatment of BV with clindamycin has been linked to the development of antimicrobial resistance among anaerobic bacteria.21 Resistance to antimicrobial therapies for N. gonorrhoeae, Mycoplasma genitalium (M. genitalium), and Candida have been well documented. 8, 21, 31 When available, antibiotic sensitivity profiles of aerobic and anaerobic vaginal pathogens in women of reproductive age should be an essential part of an assessment.
Streamline Scientific Vaginal Infections Panel
At Streamline Scientific, we provide accurate, comprehensive PCR assay for the detection of organisms comprising both normal vaginal microbiota and pathogens implicated in a wide range of vaginal infections including sexually transmitted diseases. In addition, we offer antibiotic sensitivity profiles to help clinicians develop targeted therapies that may reduce the use of broad therapeutic regimens known to contribute to the growing threat of antibiotic resistance.
General Guidelines
Laboratory test results should always be considered in the context of clinical observations and epidemiological data (such as local prevalence rates and current outbreak/epicenter locations) in making a final diagnosis and patient management decisions. With any test, the possibility of false positive and false negative results should always be considered, and the impact on patient management decisions and clinical outcomes should be carefully weighed.
References
- Acosta, C. D., Kurinczuk, J. J., Lucas, D. N., Tuffnell, D. J., Sellers, S., Knight, M., & United Kingdom Obstetric Surveillance, S. (2014). Severe maternal sepsis in the UK, 2011-2012: a national case-control study. PLoS Med, 11(7), e1001672.
- Allsworth, J. E., Lewis, V. A., & Peipert, J. F. (2008). Viral sexually transmitted infections and bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Sex Transm Dis, 35(9), 791-796. doi:10.1097/OLQ.0b013e3181788301.
- Bayar, E., Bennett, P. R., Chan, D., Sykes, L., & MacIntyre, D. A. (2020). The pregnancy microbiome and preterm birth. Semin Immunopathol, 42(4), 487-499. doi:10.1007/s00281-020-00817-w.
- Biagi, E., Vitali, B., Pugliese, C., Candela, M., Donders, G. G., & Brigidi, P. (2009). Quantitative variations in the vaginal bacterial population associated with asymptomatic infections: a real-time polymerase chain reaction study. Eur J Clin Microbiol Infect Dis, 28(3), 281-285. doi:10.1007/s10096-008-0617-0.
- Black, C. G., Tavares, L., Stachel, A., Ratner, A. J., & Randis, T. M. (2019). Distribution of Late-Onset Neonatal Sepsis Pathogens Differs in Inpatient and Outpatient Settings. Am J Perinatol, 36(11), 1136-1141. doi:10.1055/s-0038-1675643.
- Borgdorff, H., Tsivtsivadze, E., Verhelst, R., Marzorati, M., Jurriaans, S., Ndayisaba, G. F., . . . van de Wijgert, J. H. (2014). Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J, 8(9), 1781-1793. doi:10.1038/ismej.2014.26.
- Brotman, R. M. (2011). Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest, 121(12), 4610-4617. doi:10.1172/JCI57172.
- Brunham, R. C., Gottlieb, S. L., & Paavonen, J. (2015). Pelvic inflammatory disease. N Engl J Med, 372(21), 2039-2048. doi:10.1056/NEJMra1411426.
- CDC 2015 Sexually Transmitted Disease Treatment Guidelines. Retrieved from https://www.cdc.gov/std/tg2015/vaginal-discharge.htm.
- Esber, A., Vicetti Miguel, R. D., Cherpes, T. L., Klebanoff, M. A., Gallo, M. F., & Turner, A. N. (2015). Risk of Bacterial Vaginosis Among Women With Herpes Simplex Virus Type 2 Infection: A Systematic Review and Meta-analysis. J Infect Dis, 212(1), 8-17. doi:10.1093/infdis/jiv017.
- Eschenbach, D. A., Davick, P. R., Williams, B. L., Klebanoff, S. J., Young-Smith, K., Critchlow, C. M., & Holmes, K. K. (1989). Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol, 27(2), 251-256. doi:10.1128/JCM.27.2.251-256.1989.
- Fan, A., Yue, Y., Geng, N., Zhang, H., Wang, Y., & Xue, F. (2013). Aerobic vaginitis and mixed infections: comparison of clinical and laboratory findings. Arch Gynecol Obstet, 287(2), 329-335. doi:10.1007/s00404-012-2571-4.
- Gajer, P., Brotman, R. M., Bai, G., Sakamoto, J., Schutte, U. M., Zhong, X., . . . Ravel, J. (2012). Temporal dynamics of the human vaginal microbiota. Sci Transl Med, 4(132), 132ra152. doi:10.1126/scitranslmed.3003605.
- Gaydos, C. A., Beqaj, S., Schwebke, J. R., Lebed, J., Smith, B., Davis, T. E., . . . Cooper, C. K. (2017). Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet Gynecol, 130(1), 181-189. doi:10.1097/AOG.0000000000002090.
- Global incidence and prevalence of selected curable sexually transmitted infections— 2008. Geneva: World Health Organization, 2012. Retrieved from (http://www .who .int/reproductivehealth/ publications/ rtis/stisestimates/ en/ index .html).
- Haggerty, C. L., Hillier, S. L., Bass, D. C., Ness, R. B., Evaluation, P. I. D., & Clinical Health study, i. (2004). Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis, 39(7), 990-995. doi:10.1086/423963.
- Hassan, M. F., Rund, N. M. A., El-Tohamy, O., Moussa, M., Ali, Y. Z., Moussa, N., . . . Abdallah, E. A. A. (2020). Does Aerobic Vaginitis Have Adverse Pregnancy Outcomes? Prospective Observational Study. Infect Dis Obstet Gynecol, 2020, 5842150. doi:10.1155/2020/5842150.
- Jespers, V., Kyongo, J., Joseph, S., Hardy, L., Cools, P., Crucitti, T., . . . van de Wijgert, J. (2017). A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep, 7(1), 11974. doi:10.1038/s41598-017-12198-6.
- Kaambo, E., Africa, C., Chambuso, R., & Passmore, J. S. (2018). Vaginal Microbiomes Associated With Aerobic Vaginitis and Bacterial Vaginosis. Front Public Health, 6, 78. doi:10.3389/fpubh.2018.00078.
- Kim, T. K., Thomas, S. M., Ho, M., Sharma, S., Reich, C. I., Frank, J. A., . . . Wilson, B. A. (2009). Heterogeneity of vaginal microbial communities within individuals. J Clin Microbiol, 47(4), 1181-1189. doi:10.1128/JCM.00854-08.
- Kirkcaldy, R. D., Bolan, G. A., & Wasserheit, J. N. (2013). Cephalosporin-resistant gonorrhea in North America. JAMA, 309(2), 185-187. doi:10.1001/jama.2012.205107.
- Krauss-Silva, L., Almada-Horta, A., Alves, M. B., Camacho, K. G., Moreira, M. E., & Braga, A. (2014). Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multiethnic South American population. BMC Pregnancy Childbirth, 14, 107. doi:10.1186/1471-2393-14-107.
- Krohn, M. A., Thwin, S. S., Rabe, L. K., Brown, Z., & Hillier, S. L. (1997). Vaginal colonization by Escherichia coli as a risk factor for very low birth weight delivery and other perinatal complications. J Infect Dis, 175(3), 606-610. doi:10.1093/infdis/175.3.606.
- Lewis, F. M., Bernstein, K. T., & Aral, S. O. (2017). Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet Gynecol, 129(4), 643-654. doi:10.1097/AOG.0000000000001932.
- Li, J., McCormick, J., Bocking, A., & Reid, G. (2012). Importance of vaginal microbes in reproductive health. Reprod Sci, 19(3), 235-242. doi:10.1177/1933719111418379.
- Lowe, N. K., Neal, J. L., & Ryan-Wenger, N. A. (2009). Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol, 113(1), 89-95. doi:10.1097/AOG.0b013e3181909f63.
- Lynch, T., Peirano, G., Lloyd, T., Read, R., Carter, J., Chu, A., . . . Church, D. (2019). Molecular Diagnosis of Vaginitis: Comparing Quantitative PCR and Microbiome Profiling Approaches to Current Microscopy Scoring. J Clin Microbiol, 57(9). doi:10.1128/JCM.00300-19.
- MacIntyre DA, S. L., Bennett PR. (2017). The human femaleurogenital microbiome: complexity in normality. Emerging Topics in Life Sciences, 1(4), 363-372. doi:https://doi.org/10.1042/ETLS20170042.
- Menard, J. P., Mazouni, C., Fenollar, F., Raoult, D., Boubli, L., & Bretelle, F. (2010). Diagnostic accuracy of quantitative real-time PCR assay versus clinical and Gram stain identification of bacterial vaginosis. Eur J Clin Microbiol Infect Dis, 29(12), 1547-1552. doi:10.1007/s10096-010-1039-3.
- Mendling, W. (2016). Vaginal Microbiota. Adv Exp Med Biol, 902, 83-93. doi:10.1007/978-3-319-31248-4_6.
- Nasrollahi, Z., Yadegari, M. H., Roudbar Mohammadi, S., Roudbary, M., Hosseini Poor, M., Nikoomanesh, F., & Rajabi Bazl, M. (2015). Fluconazole Resistance Candida albicans in Females With Recurrent Vaginitis and Pir1 Overexpression. Jundishapur J Microbiol, 8(9), e21468. doi:10.5812/jjm.21468.
- Ness, R. B., Kip, K. E., Hillier, S. L., Soper, D. E., Stamm, C. A., Sweet, R. L., . . . Richter, H. E. (2005). A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol, 162(6), 585-590. doi:10.1093/aje/kwi243.
- Paladine, H. L., & Desai, U. A. (2018). Vaginitis: Diagnosis and Treatment. Am Fam Physician, 97(5), 321-329. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29671516. https://www.aafp.org/afp/2018/0301/afp20180301p321.pdf.
- Price, M. J., Ades, A. E., De Angelis, D., Welton, N. J., Macleod, J., Soldan, K., . . . Horner, P. J. (2013). Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol, 178(3), 484-492. doi:10.1093/aje/kws583.
- Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., . . . Forney, L. J. (2011). Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A, 108 Suppl 1, 4680-4687. doi:10.1073/pnas.1002611107.
- Reid, G. (2001). Probiotic agents to protect the urogenital tract against infection. Am J Clin Nutr, 73(2 Suppl), 437S-443S. doi:10.1093/ajcn/73.2.437s.
- Sangeetha K. T., S. G., Vasudha C. L. (2015). A study of aerobic bacterial pathogens associated with vaginitis in reproductive age group women (15-45 years) and their sensitivity pattern. Int J Res Med Sci., 3 (9), 2268–2273.
- Shipitsyna, E., Roos, A., Datcu, R., Hallen, A., Fredlund, H., Jensen, J. S., . . . Unemo, M. (2013). Composition of the vaginal microbiota in women of reproductive age–sensitive and specific molecular diagnosis of bacterial vaginosis is possible? PLoS One, 8(4), e60670. doi:10.1371/journal.pone.0060670.
- Simoes, J. A., Discacciati, M. G., Brolazo, E. M., Portugal, P. M., Dini, D. V., & Dantas, M. C. (2006). Clinical diagnosis of bacterial vaginosis. Int J Gynaecol Obstet, 94(1), 28-32. doi:10.1016/j.ijgo.2006.04.013.
- Simonsen, K. A., Anderson-Berry, A. L., Delair, S. F., & Davies, H. D. (2014). Early-onset neonatal sepsis. Clin Microbiol Rev, 27(1), 21-47. doi:10.1128/CMR.00031-13.
- St Amant, D. C., Valentin-Bon, I. E., & Jerse, A. E. (2002). Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infect Immun, 70(12), 7169-7171. doi:10.1128/iai.70.12.7169-7171.2002.
- van de Wijgert, J. (2017). The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention. PLoS Med, 14(12), e1002478. doi:10.1371/journal.pmed.1002478.
- van de Wijgert, J., & Jespers, V. (2017). The global health impact of vaginal dysbiosis. Res Microbiol, 168(9-10), 859-864. doi:10.1016/j.resmic.2017.02.003.
- van de Wijgert, J., Verwijs, M. C., Gill, A. C., Borgdorff, H., van der Veer, C., & Mayaud, P. (2020). Pathobionts in the Vaginal Microbiota: Individual Participant Data Meta-Analysis of Three Sequencing Studies. Front Cell Infect Microbiol, 10, 129. doi:10.3389/fcimb.2020.00129.
- van de Wijgert, J. H., Borgdorff, H., Verhelst, R., Crucitti, T., Francis, S., Verstraelen, H., & Jespers, V. (2014). The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One, 9(8), e105998. doi:10.1371/journal.pone.0105998.
Ready to take charge of your lab
services?
START YOUR LAB
USE OUR LAB
© Copyright 2025 | All Rights Reserved | Privacy Policy | Terms of Use | Do not sell or share my personal information